Formosa Pharmaceuticals Technology
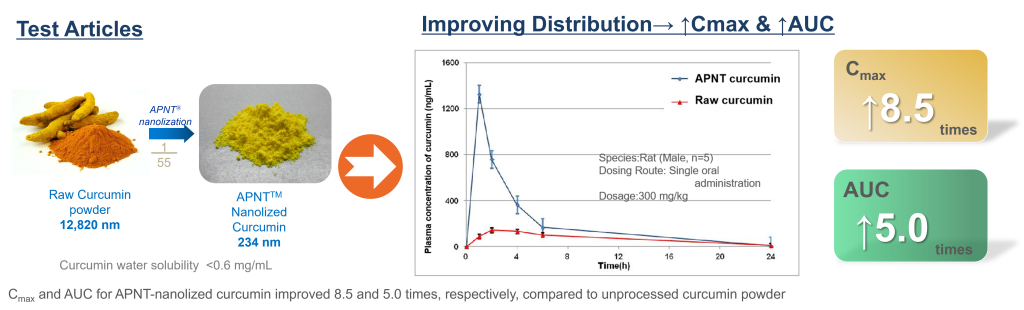
The APNT® Technology’s ability to improve bioavailability was verified by this study. We obtained common curcumin powder on the market as the test article. The particle size of the curcumin powder was about 13 micron. After APNT® nanolization process, the mean diameter was reduced to 234 nm, 1/55 of the original size.
A rat PK study was conducted to compare the concentration of curcumin in blood before and after nanolization. The study result shows that the concentration of APNT® nanolized curcumin powder group was markedly higher than the unprocessed curcumin powder. Cmax is increased by 8.5 times; the AUC is increased by 5 times.
Enhance Formulation Homogeneity and Stability through APNT® |APP13007
APP13007 is a demonstration of APNT® technology. APNT® technology enables the nanosuspension eye drops to present a superior formulation quality compared to other commercialized suspension eyedrops.
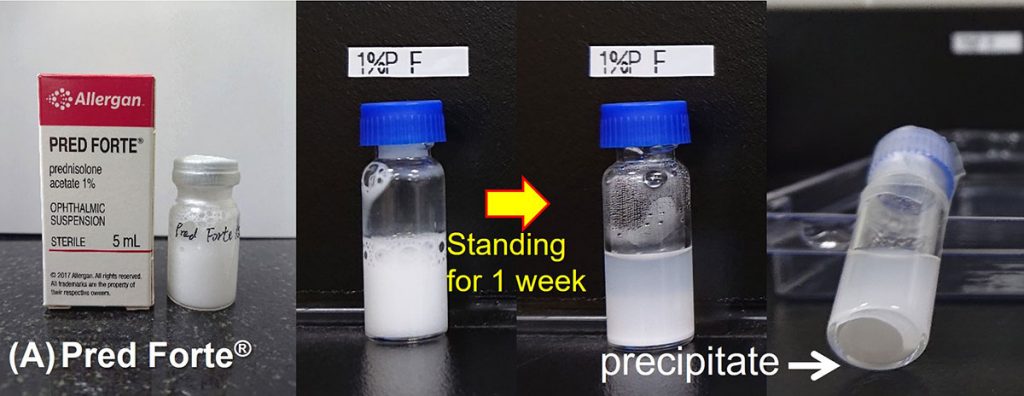

For those marketed suspension eye drops, insoluble API will precipitate after standing for a period of time (Figures A and B), which could complicate uniform instillation into the eyes. Therefore, before use, it needs to be shaken vigorously to make the API dispersed again in the solution.

Some suspension eyedrops are formulated as emulsions so the eye drops appear milky and may blur vision (Figure C).
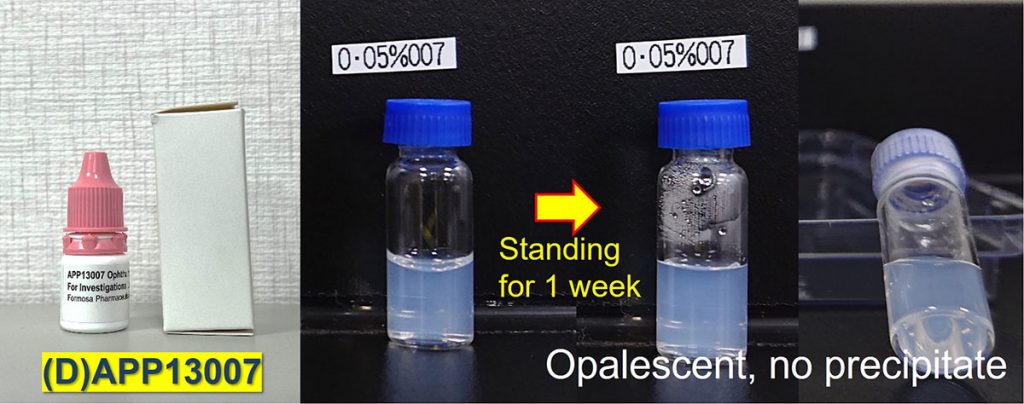
APNT® technology reduces the particle size, and thus APP13007 is uniformly dispersed in the drug product, which maintains a superior dispersion state after a week of standing (Figure D).
With these advantages, APNT® provides patients a more comfortable eye instillation experience since the formulation feels similar to water.
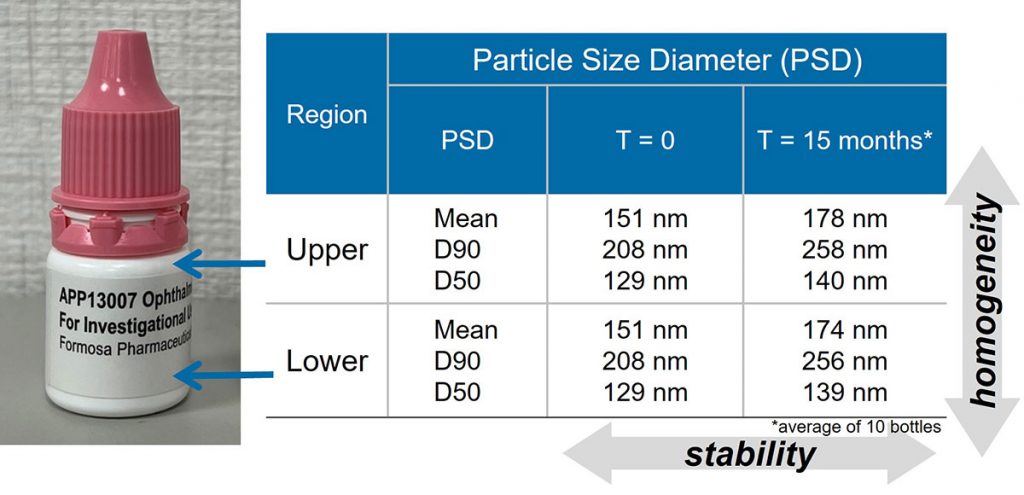
In the experiment of long-term storage at room temperature (25°C / 40% RH), the particle size distribution of the APP13007 preparation remains homogenous after 15 months of standing, and there is no layering phenomenon of upper and lower layers.
Enhance Distribution for DPI through APNT™
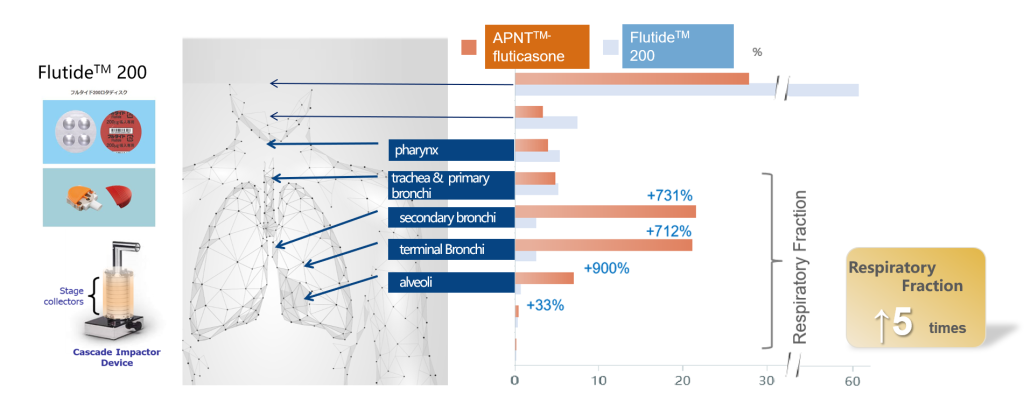
The study was conducted with a Cascade Impactor, which simulates particle distribution in the lung. We used APNT® nanolized fluticasone and Flutide™ 200 (Fluticasone Dry Powder Inhaler) as test article. The simulation shows that the APNT® nanolized fluticasone has a 5-times higher distribution in overall respiratory fractions than Flutide™ 200. In the trachea, primary bronchi, secondary bronchi and terminal bronchi and other peripheral regions measured, the distribution of drug is 7 to 9 times higher than that of Flutide™ 200 and implied less drug loss in non-respiratory fractions, such as the throat.
*Respiratory fractions include the pharynx, trachea, primary bronchi, secondary bronchi, terminal bronchi and alveoli.