Formosa Pharmaceuticals Partnering
Partnering and Collaboration
Business Development Activities

Formosa Pharmaceuticals embraces a flexible model of partnership and is dedicated to maximizing the potential and the value of the relationship for both us and our partners. We do so through an enthusiastic and clear strategic view, with comprehensive coverage from drug development expertise, technology, and solid value chain in manufacture and quality.
Partner for Ophthalmology Asset|APP13007-Inflammation and Pain Treatment after Cataract Surgery
- Formosa Pharma entered Licensing Agreements with:
- Eyenovia for United States and territories
- China Grand Pharmaceutical for China, Hong Kong, and Macau
- Cristalia Produtos Quimicos Farmaceuticos Ltda for Brazil
- Tabuk Pharmaceuticals Manufacturing Company for MENA (Middle East and North Africa) market
- Tzamal Biopharma Ltd. for Israeli market
- Apotex Inc. for Canadian market
- DÁVI Farmacêutica for Portuguese Market
- Medvisis Switzerland AG for Swiss Market
- Cipla Limited for 11 countries including India, Nepal, Sri Lanka, Bangladesh, Malaysia, Myanmar, Kenya, Nigeria, South Africa, Argentina, and Colombia.
- For other countries and regions outside aforementioned territories, we welcome specialty pharmaceutical companies to discuss collaboration opportunities with us.
Strategic Partner
Eyenovia, Inc. (NASDAQ: EYEN) is a commercial stage ophthalmic pharmaceutical technology company developing a pipeline of microdose array print therapeutics. Eyenovia is currently focused on the commercialization of Mydcombi™ for mydriasis, as well as the ongoing late-stage development of medications in the Optejet® device for presbyopia and myopia progression. Formosa Pharmaceuticals has entered into a licensing agreement in 2023, whereby Eyenovia obtains exclusive U.S. rights for the commercialization of APP13007 (clobetasol propionate ophthalmic nanosuspension, 0.05%) for the treatment of inflammation and pain following ocular surgery.
For more information, visit www.eyenovia.com.
Grand Pharmaceutical Group Co., Ltd. (stock code: 00512. HK) is a research-based international pharmaceutical enterprise that specializes in pharmaceutical technology, diagnosis and treatment technology and biotechnology.
Grand Pharma has marketed nearly 30 products in the ophthalmology sector, covering chemical drugs, traditional Chinese medicine and eye health products, including prescription drugs, OTC, devices, consumer goods, etc., to create a “public eye care ecosystem” which integrates “prevention + treatment + health care”. Formosa Pharmaceuticals has entered into a licensing agreement in 2021, whereby Grand Pharma obtains exclusive Greater China rights (China, Hong Kong, Macau) for the commercialization of APP13007 (clobetasol propionate ophthalmic nanosuspension, 0.05%) for the treatment of inflammation and pain following ocular surgery.
For more information, visit https://www.grandpharm.com/en/.
Established over 50 years, Cristália Laboratory is a 100% Brazilian Pharmaceutical, Pharmochemical, Biotechnological, Research/Development/Innovation-based Industrial Complex. It is a pioneer in thoroughly executing a drug production chain, from its molecule design to the finished product. The company holds 127 patents – a national record – and is a market leader in anesthesia in Latin America. Occupying a forefront position when it comes to innovation, Cristália produces 53% of the raw materials of its drugs at its Pharmacochemical plant. The company is present in over 95% of Brazilian hospitals, being an international reference in active substances and finished products exportation to over 30 countries. For the last 20 years, the company has been investing in biotechnology, owning some of Brazil’s first private Biotech plants. Cristália Group has about 5,000 colleagues.
For more information, visit https:// www.cristalia.com.br/.
Established in 1994, Tabuk Pharmaceuticals is a leading Saudi pharmaceutical company with a regional presence in the Middle East and North Africa (MENA). Tabuk Pharmaceuticals develops, manufactures, markets, and distributes various branded generics, in addition to manufacturing pharmaceutical products for renowned international partners at its manufacturing sites in Saudi Arabia, as part of its continuous efforts to cover the needs of patients by providing high quality medicines. Tabuk Pharmaceuticals is a major player in the pharmaceutical sector thanks to its four state-of-the-art manufacturing sites located in Tabuk and Dammam in the Kingdom, as well as in Sudan and Algeria, orchestrated by a team of more than 2,400 employees. Tabuk Pharmaceuticals reaches patients in 17 countries across the Middle East and North Africa, with future plans to expand its presence in the region.
For more details about Tabuk Pharmaceuticals Manufacturing Company https://www.tabukpharmaceuticals.com
For more details about Tzamal BIO-Pharma https://tzamal-biopharma.co.il/
Apotex is a Canadian-based global health company. We improve everyday access to affordable, innovative medicines and health products for consumers worldwide, with a broad portfolio of generic, biosimilar, and innovative branded pharmaceutical products. Headquartered in Toronto, with regional offices globally, including in the United States, Mexico and India, we are the largest Canadian-based pharmaceutical company and a health partner of choice for the Americas for pharmaceutical licensing and product acquisitions. For more details about Apotex Inc. www.apotex.com.
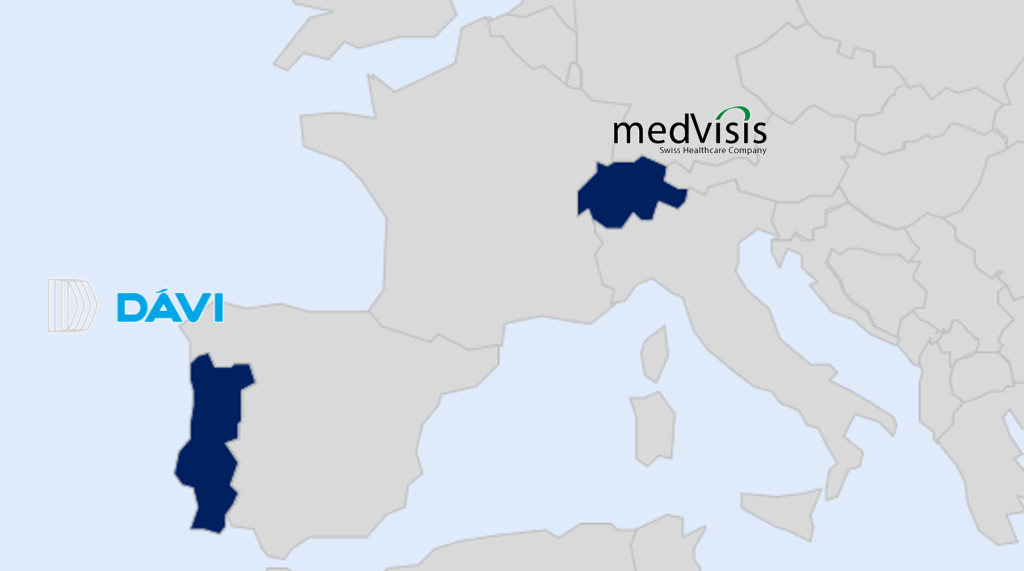
DÁVI Farmacêutica, is a century-old company and one of the key players in ophthalmology and ENT segments in Portugal. The company’s core activities are the distribution & promotion of branded medicines with significant partnerships with major multinational pharmaceutical companies. Our daily mission is to give our patients the best option available in the market, making their lives better. To know more about DÁVI Farmacêutica, visit www.davi.pt/en.
Medvisis Switzerland AG is the partner of choice for innovative specialty, rare disease, and hospital products in Switzerland with expertise in product registration, reimbursement and market access, and rapid commercialization uptake. The company targets the in-licensing of pharmaceutical products for which there is high medical need along with strong endorsement from key opinion leaders. To know more about Medvisis, visit www.medvsis.com.
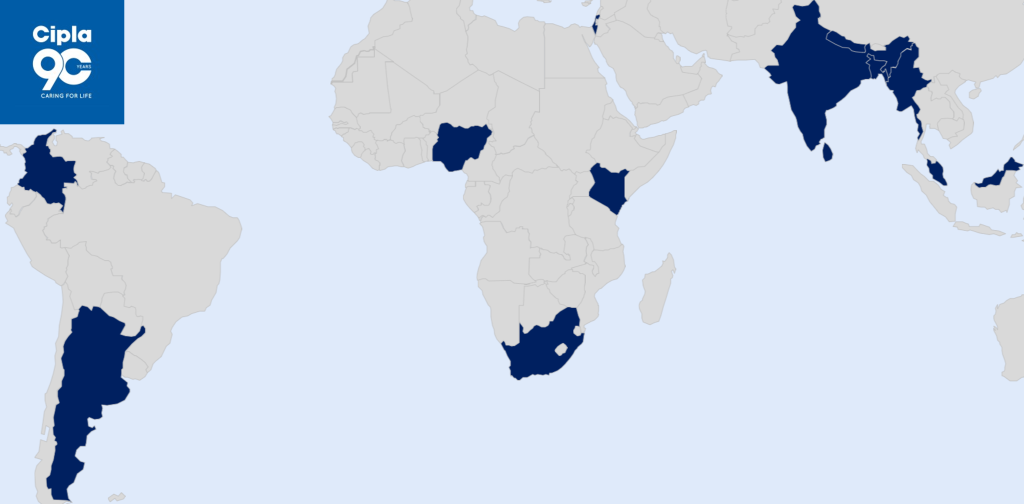
Established in 1935, Cipla is a global pharmaceutical company focused on agile and sustainable growth, complex generics, and deepening portfolio in our home markets of India, South Africa, North America, and key regulated and emerging markets. Our strengths in the respiratory, anti-retroviral, urology, cardiology, anti-infective and CNS segments are well-known. Our 46 manufacturing sites around the world produce 50+ dosage forms and 1,500+ products using cutting-edge technology platforms to cater to our 80+ markets. Cipla is ranked 3rd largest in pharma in India (IQVIA MAT Dec’24), largest in the pharma prescription market in South Africa (IQVIA MAT Nov’24), and 4th largest by prescription in the US Gx (Repulses + MDI) products (IQVIA MAT Nov’24). For over eight decades, making a difference to patients has inspired every aspect of Cipla’s work. Our paradigm-changing offer of a triple anti-retroviral therapy in HIV/AIDS at less than a dollar a day in Africa in 2001 is widely acknowledged as having contributed to bringing inclusiveness, accessibility and affordability to the centre of the HIV movement. A responsible corporate citizen, Cipla’s humanitarian approach to healthcare in pursuit of its purpose of ‘Caring for Life’ and deep-rooted community links wherever it is present make it a partner of choice to global health bodies, peers and all stakeholders. For more, please visit www.cipla.com To know more about Cipla, visit www.cipla.com.
Partner for Ophthalmology Asset|APP13002-for Infectious Eye Diseases treatment

APP13002 is in IND-enablement stages and is available for licensing or co-development either regionally or globally.
We welcome regional specialty pharmaceutical companies to discuss cooperation opportunities with us.
We are seeking partners who are interested in China-US dual registration.
Partner for Oncology Biosimilar Asset|TSY-0110 Biosimilar Kadcyla®
TSY-0110 is flexible to work with the partners’ strategy.
Co-development with partners in the scope of a Global Phase 3 study.
Leveraging in integration and manufacturing advantages to ensure successful CMC development, regulatory registration and scalability for TSY-0110.
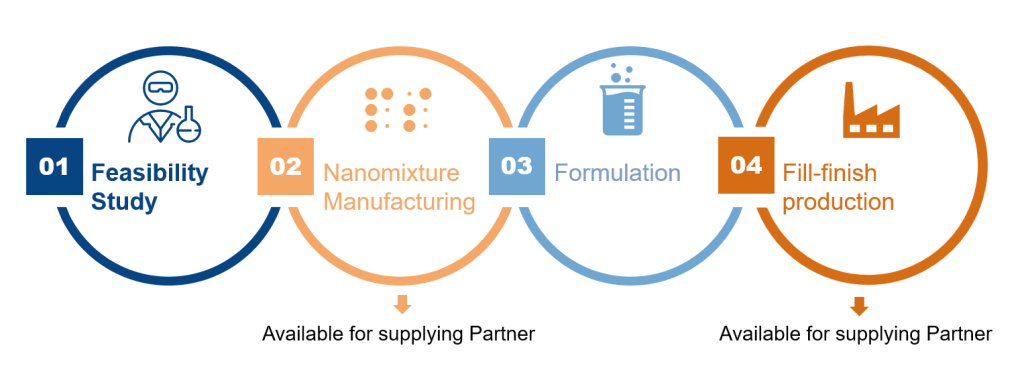
Collaborate with Formosa Pharmaceuticals:
Formosa Pharmaceuticals is open to collaborations with biopharmaceutical companies who have face challenges in formulation of APIs with poor solubility and / or absorption.
Feasibility Study (conducted in 2-3 months).
Formosa Pharmaceuticals is flexible to work with partners in providing nanomixture or manufacture the formulation and fill-finish production to provide finished products to the partners.